The “basal ganglia” refers to a group of subcortical nuclei within the brain responsible primarily for motor control, as well as other roles such as motor learning, executive functions, emotional behaviours, and play an important role in reward and reinforcement, addictive behaviours and habit formation.
The basal ganglia are located at the base of the forebrain (cerebrum) and have attracted attention in medicine for various disturbances that appear with dysfunctions caused by diseases or trauma. Disruption of the basal ganglia network forms the basis for several movement disorders eg Parkinson's Disease, Huntington Disease.
 |
| Basal Ganglia |
Proposed more than two decades ago, the classical basal ganglia model shows how information flows through the basal ganglia back to the cortex through two pathways with opposing effects for the proper execution of movement.
Much of the model has remained today however the model has been modified and amplified with the emergence of new data. The basal ganglia network is now viewed as multiple parallel loops and re-entering circuits whereby motor, associative, and limbic territories are engaged mainly in the control of movement, behaviour, and emotions. These parallel circuits subserve the other functions of the basal ganglia engaging associative and limbic territories.
Structure
The basal ganglia are a cluster of subcortical nuclei deep to cerebral hemispheres. The largest component of the basal ganglia is the corpus striatum which contains the caudate and lenticular nuclei (the putamen, globus pallidus externus, and internus), the subthalamic nucleus (STN), and the substantia nigra (SN). These structures intricately synapse onto one another to promote or antagonize movement.
Divisions of the Basal Ganglia ie subcortical nuclei.
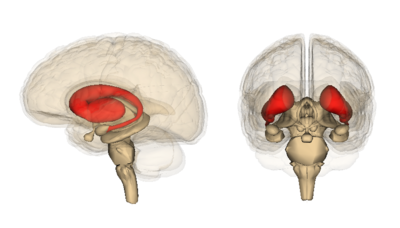
- Corpus Striatum- (The largest subcortical brain structure of the basal ganglia is the striatum with a volume of approximately 10 cm). It is a heterogeneous structure that receives afferents from several cortical and subcortical structures and projects to various basal ganglia nuclei. Within the striatum, there are two main divisions
- Dorsal striatum (DS) see image, shown in red. Primarily involved in control over conscious motor movements and executive functions. The dorsal striatum consists of the caudate nucleus and the putamen. A white matter, nerve tract (the internal capsule) in the dorsal striatum separates the caudate nucleus and the putamen.
- Ventral striatum, responsible for limbic functions of reward and aversion. Consists of nucleus accumbens and the olfactory tubercle.
- Internal and External segments of Globus Pallidus (NB until the first half of the 19th century the globus pallidus and putamen were considered one structure, collectively referred to as the lentiform or lenticular nucleus)
- Subthalamic Nucleus (STN) - a lens-shaped cell group that makes up the largest part of the subthalamus
- Substantia Nigra (SN) - (“black substance” in Latin) is a long nucleus located in the midbrain but considered functionally a part of the basal ganglia because of its reciprocal connections with other brainstem nuclei. It consists of two components, the pars compacta and the pars reticulata, which have different connections and use different neurotransmitters. The 2 minute video below outlines BG concepts
Basal Ganglia - Current Concepts
Main circuits of the basal ganglia are shown below. (Very complicated and involved circuits exist)The original functional organization of the basal ganglia was conceived as a loop, in which cortical afferent activity is dispatched to and modulated by the basal ganglia, which subsequently sends back a signal to the cortex to facilitate (or inhibit) motor activity. The basal ganglia were featured as a “go through” station within the motor loop. Current thinking now is that the basal ganglia has several loops, where cortical and subcortical projections interact with internal reentry loops forming a complex network, ideally designed for selecting and inhibiting simultaneously occurring events and signals.
Coronal slices that have been superimposed to include the involved basal ganglia structures. + and - signs at the point of the arrows indicate respectively whether the pathway is excitatory or inhibitory in effect. Green arrows refer to excitatory glutamatergic pathways, red arrows refer to inhibitory GABAergic pathways and turquoise arrows refer to dopaminergic pathways that are excitatory on the direct pathway and inhibitory on the indirect pathway. Note that dis-inhibitory pathways in effect are excitatory on the feedback to the cortex, while dis-dis-inhibitory pathways in effect are inhibitory.
Pathophysiology
The basal ganglia are particularly associated with movement disorders. Associated with damage to the BG are: tremors; involuntary muscle movements; abnormal increase in tone; difficulty initiating movements; abnormal posture.
Movement disorders comprise a variety of motor problems, not all of which are associated with dysfunction of the basal ganglia. Those that have a clearly established pathological basis and are caused by pathophysiological mechanisms directly involving the basal ganglia include
Parkinson's
Parkinson's is the most notorious disease of the basal ganglia. Classic clinical symptoms include bradykinesia, resting tremor, postural instability, and shuffling gait. This disease is a result of neurodegeneration of the SNpc dopaminergic neurons. Often found in the Parkinsonian striatum, alpha-synuclein protein aggregates form toxic “Lewy bodies,” which are inclusions within neurons. The substantia nigra, due to degeneration, loses its grossly visible dark pigmentation, a concomitant sign of dopamine biosynthesis dysfunction. This loss of dopamine depresses the nigrostriatal pathway. With decreased dopaminergic input the striatum exerts less positive motor activity and more negative motor inhibition. This gives the characteristic hypokinetic dysfunction found in these patients.
Huntington Disease
Huntington disease is a hyperkinetic movement disorder. Its cause is a genetic defect manifesting as a CAG repeat on chromosome 4p on the HTT gene. This creates an abnormally long Huntington gene which leads to neuronal death in the caudate and the putamen. The indirect pathway is interrupted and leads to a hyperkinetic presentation. Symptoms include involuntary movements such as chorea, cognitive degeneration, and psychiatric dysfunction.
Hemiballism
Hemiballism (from the Greek “to throw”) is used to describe hyperkinetic, involuntary, forceful movements of the ipsilateral arm and leg. Commonly, a lesion in the contralateral subthalamic nuclei causes hemiballism. Given that the subthalamus is part of the indirect pathway this lesion reduces or eliminates indirect pathway signalling, leading to a relative overabundance of activity in the direct pathway. Such causes include stroke, traumatic brain injury, amyotrophic lateral sclerosis, nonketotic hyperglycemia, neoplasm, vascular malformation, and other causes.
Tourette Syndrome
Tourette syndrome has been shown to have a significant neurological basal ganglia component which manifests as sudden, repetitive uncontrolled movements and vocalizations, called “tics.” These tics have been associated with dysfunction of the GABAergic projections from the striatum, leading to a relative increase in dopaminergic activity much like in hemiballism and Huntington’s disease
Additionally, parts of the basal ganglia play a key role in reward and reinforcement, addictive behaviours and habit formation. Pathophysiological processes underlying psychiatric disorders such as depression and obsessive-compulsive disorder involve the basal ganglia and their connections with many other structures (particularly to the prefrontal cortex and the limbic system). In terms of cognitive disorders, basal ganglia abnormalities have been found in individuals with schizophrenia and may explain have learning deficits associated with the disorder.
Silent Cerebral Infarcts - of interest a 2008 study found that approx. 5% of healthy middle-aged adults have microlesions in their BG.
Physiotherapy - Implications From Recent Studies
The importance and value of exercise are becoming more and more apparent for a whole raft of health conditions. Here are a few of the latest findings re exercise and BG function.
- A 2016 study by Becker et al. into cognitive performance and BG changes concluded that physical activity, especially motor fitness level training, might be a promising tool that leads to structural changes in the basal ganglia. This might have the potential to diminish the cognitive decline in older adults and to support academic success in children and young adults.
- Exercise is beneficial and should be routinely prescribed in the management of PD (may aid in BG function).
- Exercise effects on basal ganglia damage
- The adult brain possesses a tremendous capacity for experience-dependent neuroplasticity, even in the context of ageing and neurodegenerative disorders including PD, where the activation of neurotrophic factors may play a key role.
- Animal models of dopamine depletion are beginning to reveal the underlying mechanisms by which exercise can remodel the brain through alteration in neuronal synaptic connections, especially dopaminergic and glutamatergic neurotransmission within the basal ganglia.
- Epidemiological studies, clinical observations, and animal research indicate that appropriately dosed physical activity and exercise may not only reduce the risk of developing PD in vulnerable populations but also benefit PD patients by potentially protecting the residual nigrostriatal dopamine neurons or directly restoring the dysfunctional cortico-basal ganglia motor control circuit, and these benefits may be mediated by exercise-triggered production of endogenous neuroprotective molecules such as neurotrophic factors.
- Animal studies have been instrumental in providing evidence for exercise-induced neuroplasticity of corticostriatal circuits that are profoundly affected in Parkinson’s disease. Exercise has been implicated in modulating dopamine and glutamate neurotransmission, altering synaptogenesis, and increasing cerebral blood flow. In addition, recent evidence supports that the type of exercise may have regional effects on brain circuitry, with skilled exercise differentially affecting frontal-striatal related circuits to a greater degree than pure aerobic exercise (with skilled exercise differentially affecting frontal related circuits more so than pure aerobic exercise). These effects are not mutually exclusive eg peddling on a recumbent bicycle may be considered predominantly aerobic with minimal skill or cognitive engagement whilst at the other end of the spectrum juggling may represent a highly skilled task with minimal aerobic involvement. However, many exercises such as swimming and running involve a combination of both skilled and aerobic exercise. These results indicate that the community Parkinson Dance classes that exist are a great option, as are many other structured classes.


0Comments